
Medicines for Ireland are delighted to announce the publication of new Code of Practice
Username or Email Password Remember me Lost your password?

Username or Email Password Remember me Lost your password?
PRESS RELEASE 5 May 2022 Value Added Medicines (VAMs) are medicines based on known molecules that address healthcare needs and deliver relevant improvements for patients

PRESS RELEASE 13th April 2022 EU ensures uninterrupted supply of medicines between Ireland and the UK New measures ensure sustained supply of medicines from Great

The supply of medicines is critical yet unstable as war continues in Ukraine. As a key medicines supplier, our sector is doing everything possible to

Medicines for Europe is highly concerned by the impact of the war in Ukraine on the supply of medicines to patients. The priority of Medicines

Medicines for Ireland has announced the appointment of Managing Director of Accord Healthcare Ireland, Padraic O’Brien as new Chairperson of the organisation, with Paul Neill,
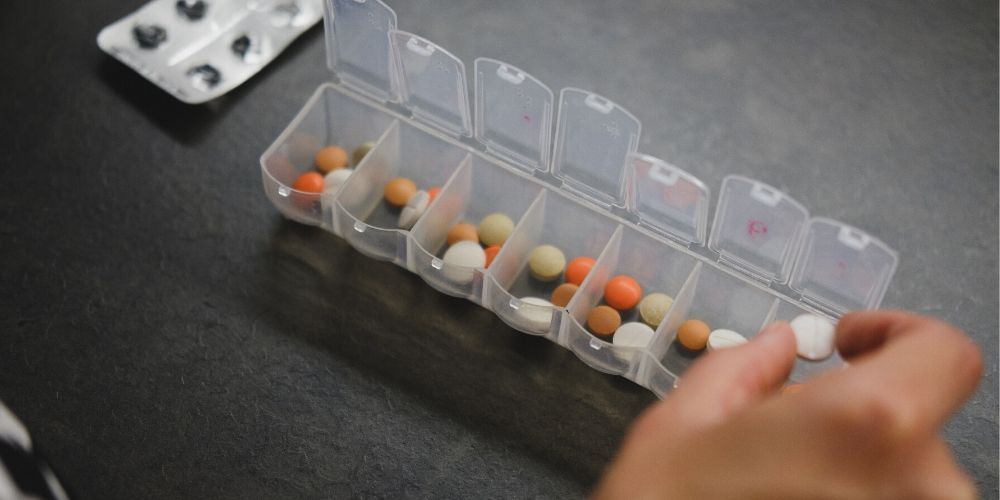
In December 2021, Medicines for Ireland (MFI) signed a new Framework Agreement on Supply and Pricing of Medicines 2021-2025 with the Department of Health, the

22nd December 2021 The Minister for Public Expenditure and Reform Michael McGrath TD has “welcomed the substantial cost efficiencies that have been secured as part

Response from Medicines for Ireland (MFI) to new Framework Agreement on Supply and Pricing of Medicines 2021-2025 ‘We welcome the new Supply and Pricing

15th December 2021 Medicine Pricing Agreements a boost for patients and good news for Irish Pharma Sector – Tánaiste and Minister for Enterprise Tánaiste

We are committed to working with key stakeholders to affect real change and reforms that ensure Irish patients receive the best medical treatment in the most affordable way.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |